
Each radioactiveĮlement, including strontium, constantly gives off radiation,Īnd this process changes it into an isotope of another element 90Sr is formed in nuclear reactors orĭuring the explosion of nuclear weapons.
Hazardous of the radioactive isotopes of the chemical element Paint pigments, fluorescent lights, medicines, and other products. Strontium compounds, such as strontium carbonate,Īre used in making ceramics and glass products, pyrotechnics,
#SR ELEMENT COMMON ION SERIES#
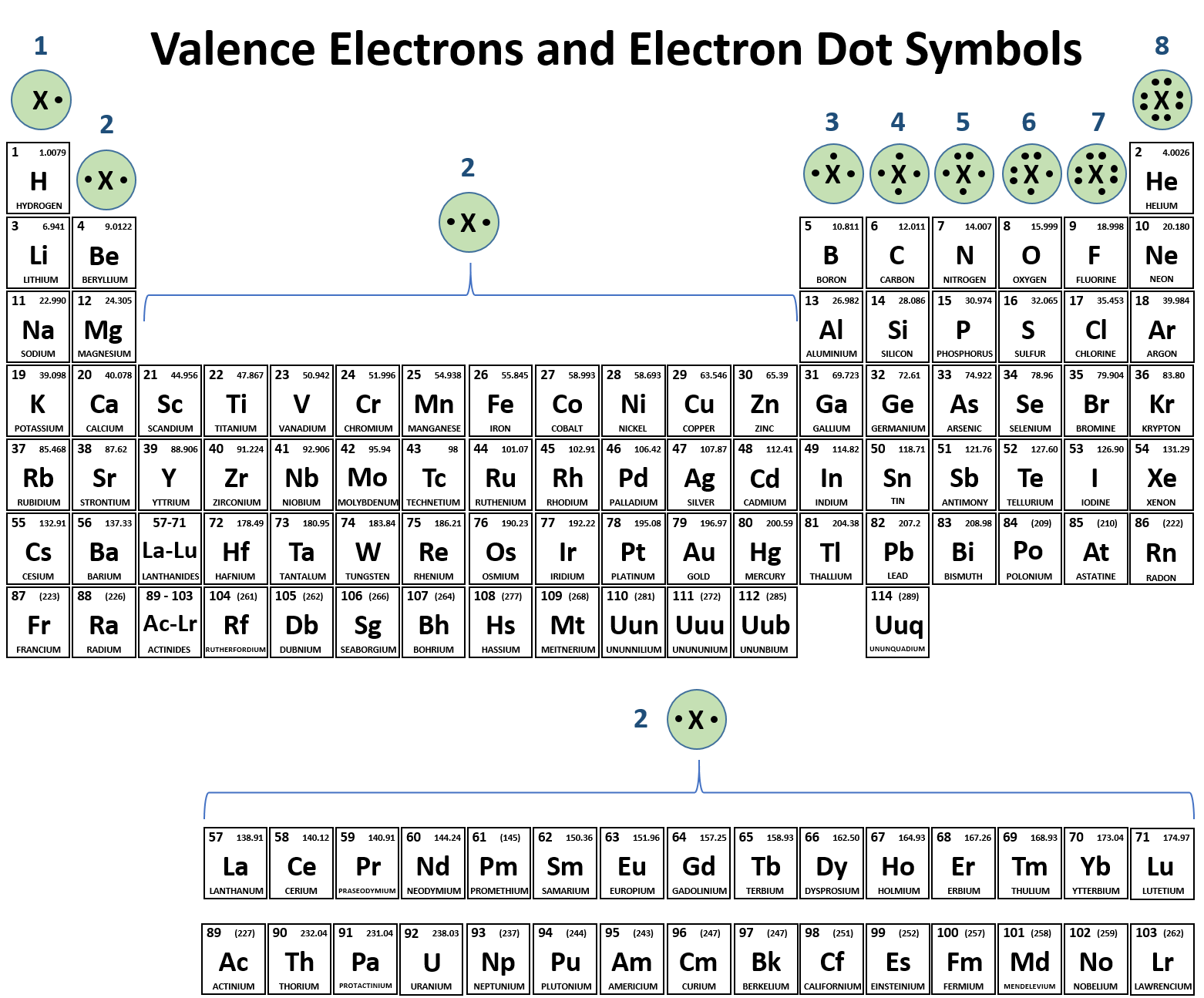
After the strontium isĮxtracted from strontium ore, it is concentrated into strontiumĬarbonate or other chemical forms by a series of chemical Is found in nature as the minerals celestite (SrSO 4)Īnd strontianite (SrCO 3). Materials are a few parts per million (ppm). So any combination of the four would have the same chemicalĪnd underground water, air, plants, and animals all contain All four isotopes behave the same chemically, Natural strontium is not radioactiveĪnd exists in four stable types (or isotopes), each of whichĪnd 88Sr, and read as strontium eighty-four, strontiumĮighty-six, etc. ThereĪre two types of strontium compounds, those that dissolve Strontium compounds do not have any particular smell. Strontium can form a variety of compounds. Rather, strontium is usually found in nature Pure strontium isĪ hard, white-colored metal, but this form is not found in Under normal environmental conditions, only the +2 oxidation Strontium can exist in two oxidation states: 0 and Strontium is a natural and commonly occurringĮlement. You must also consider the other chemicals you're exposed to and your age, sex, diet, family traits, lifestyle, and state of health. These factors include the dose (how much), the duration (how long), and how you come in contact with it. If you are exposed to strontium, many factors determine whether you'll be harmed. Man-made sources of radioactive materials are found in consumer products, industrial equipment, atom bomb fallout, and to a smaller extent from hospital waste and nuclear reactors. Naturally occurring sources of radiation are cosmic radiation from space or radioactive materials in soil or building materials.
#SR ELEMENT COMMON ION SKIN#
You may be exposed by breathing, eating, or drinking the substance, or by skin contact.Įxternal exposure to radiation may occur from natural or man-made sources. You are exposed to a substance only when you come in contact with it. This release does not always lead to exposure. When a substance is released from a large area, such as an industrial plant, or from a container, such as a drum or bottle, it enters the environment. This information is important because exposure to strontium and strontium-90 may harm you and because these sites may be sources of exposure.

As more sites are evaluated, the sites at which strontium and strontium-90 are found may increase. However, the total number of NPL sites evaluated for strontium and strontium-90 are not known. Strontium and strontium-90 have been found in at least 102 and 12 of the 1,636 current or former NPL sites, respectively.
These sites make up the National Priorities List (NPL) and are the sites targeted for long-term federal cleanup activities. The Environmental Protection Agency (EPA) identifies the most serious hazardous waste sites in the nation. This public health statement tells you about strontium and the effects of exposure. For more information, call the ATSDR Information Center at 1-80. The effects of exposure to any hazardous substance depend on the dose, the duration, how you are exposed, personal traits and habits, and whether other chemicals are present. This information is important because this substance may harm you. Health Statements about hazardous substances and their healthĮffects. What recommendations has the federal government made to protect human health?.Is there a medical test to determine whether I have been exposed to strontium?.How families reduce the risk of exposure to strontium?.How can strontium enter and leave my body?.What happens to strontium when it enters the environment?.


 0 kommentar(er)
0 kommentar(er)
